Explain Why an Empirical Formula Can Represent Many Different Molecules
Because the empirical formula just gives you the ratios of the number of atoms of each element in the compound but if that compound exists as discrete molecules the numbers of atoms of. Glucose is an important simple sugar that cells.
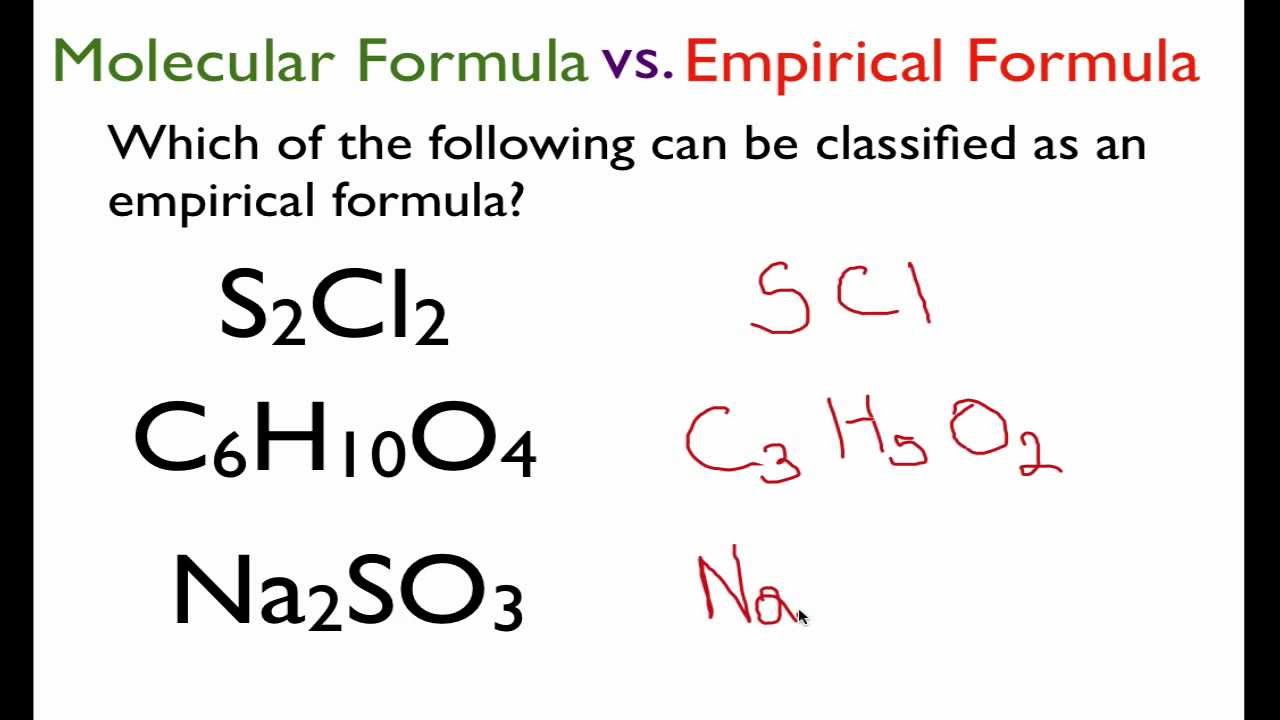
Chemical Formulas Boundless Chemistry
For Acetylene the empirical formula is CH.
. For Acetylene the empirical formula is C2H2. The empirical chemical formula represents the relative number of atoms of each element in the compound. They have the same molecular formula but.
The molecular formula shows the exact number of different types of atoms present in a molecule of a compound. Molecules with the same molecular formula can be different because their atoms are connected in different orders. One molecular formula can represent multiple structural formulas simply due to the formation of ice Immers.
The most common form of nylon Nylon-6 contains 6368 carbon 1238. Empirical formulas show the number of atoms of each element in a compound in the most simplified state using whole. Chemistry deals with specific elements to create.
Some compounds like water have the same empirical and. While molecular formula expresses the actual number of each element. An empirical formula shows the most basic form of a compound.
Start studying Chemistry Chapter 3. Molecular Formulas and Structural Formulas. Because the empirical formula is a ratio different combinations of elements could essentially have the same empirical formula.
Empirical formula is a comparison of the number of moles of a compound so you need your values in moles. An empirical formula like a molecular formula lacks any structural information about the positioning or bonding of atoms in a molecule. Empirical formula expresses the simplest mole ratio of the elements in a compound or molecule.
Learn vocabulary terms and more with flashcards games and other study tools. Empirical formulas are the simplest form of formulas that we can write for a molecule while molecular formulas are the formulas showing the type of atoms and number of. Using the oxygen example again there are 160 grams per mole of.
An empirical formula represents the simplest whole-number ratio of various atoms present in a compound. For example the molecular formula of glucose is C 6 H 12 O 6 but the empirical formula is CH 2 O. Explain why one molecular formula can represent more than one structural formula.
In this example we are calculating the empirical formula for mass composition. The molecular formula shows the actual number of atoms of each type of element. As the name suggests an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula.
For butane and isobutane the empirical formula for both molecules is C 2 H 5 and they share. So for example here we have two structural formulas for two. The empirical formula is distinct from the molecular formula in that it represents the simplest ratio of atoms involved in the compound whereas the molecular formula.
The empirical formula shows the simplest whole-number ratio between atomsions in a compound. The molecular formula is a MULTIPLE of the empirical formula and of course that. This is because we can divide each number in C 6 H 12 O 6 by 6 to make a simpler whole.
If the molecular or molar mass of the substance is known it may be divided by the empirical formula mass to yield the number of empirical formula units per molecule n. It can therefore describe a number of different structures or isomers with varying physical properties. If we know the molecular or molar mass of.
An empirical formula is a formula that shows the elements in a compound in their lowest whole-number ratio. Molecular formulas contain no information about the. The empirical formula is the simplest whole number ratio that represents constituent atoms in a species.

Molecular Formulas And Nomenclature
What Does The Empirical Formula Of A Compound Describe Quora
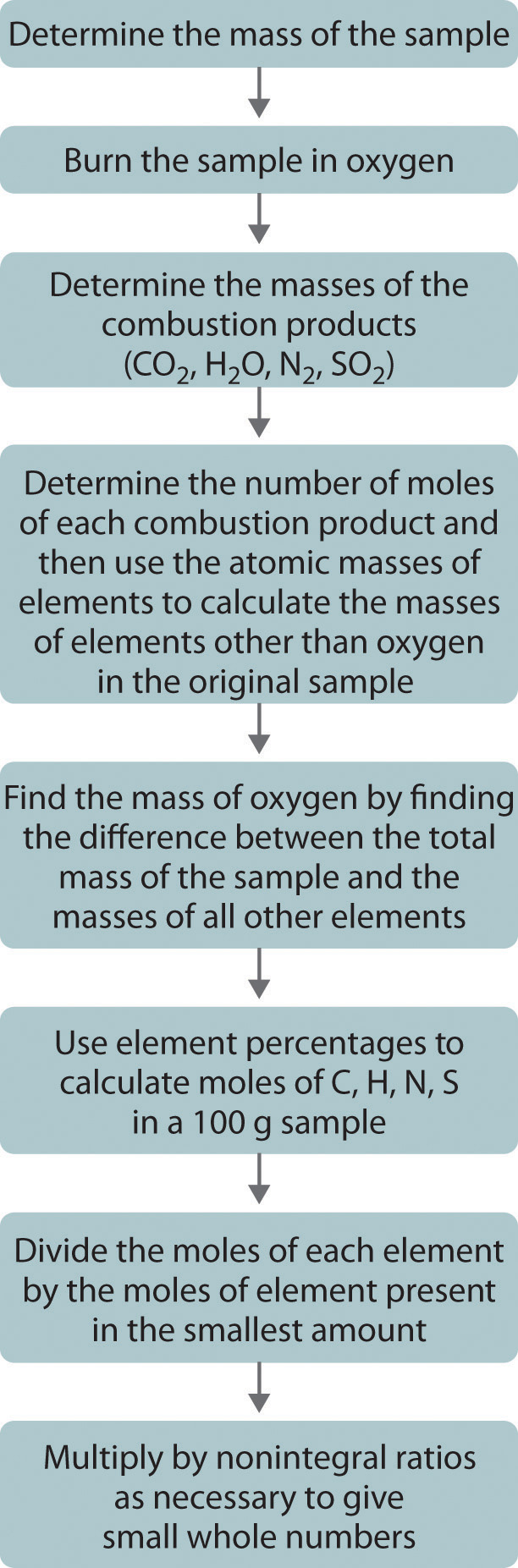
1 16 Molecular Formulas And Empirical Formulas Review Chemistry Libretexts

Empirical Formula Definition Steps Examples Video Lesson Transcript Study Com
0 Response to "Explain Why an Empirical Formula Can Represent Many Different Molecules"
Post a Comment